|
Education |

|
Glutathione transferases (GSTs) comprise a family of eukaryotic and prokaryotic metabolic isoenzymes best known for their ability to catalyze the conjugation of the reduced form of glutathione (GSH) to xenobiotic substrates for the purpose of detoxification. GSTs catalyze the conjugation of GSH — via a sulfhydryl group — to electrophilic centers on a wide variety of substrates in order to make the compounds more soluble. This activity detoxifies endogenous compounds and enables the breakdown of xenobiotics. GSTs may also bind toxins and function as transport proteins, which gave rise to the early term for GSTs. Cytosolic GSTs are dimeric, with both subunits being from the same class of GSTs, although not necessarily identical. The monomers are approximately 25 kDa in size. They are active over a wide variety of substrates with considerable overlap.
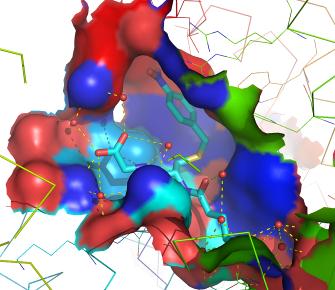
The GST family consists of three superfamilies: the cytosolic, mitochondrial, and microsomal—also known as MAPEG—proteins. Members of the GST superfamily are extremely diverse in amino acid sequence and a large fraction of the sequences deposited in public databases are of unknown function. The glutathione binding site, or "G-site," is located in the thioredoxin-like domain of both cytosolic and mitochondrial GSTs. The region containing the greatest amount of variability between the assorted classes is that of helix α2, where one of three different amino acid residues interacts with the glycine residue of glutathione. Two subgroups of cytosolic GSTs have been characterized based upon their interaction with glutathione: the Y-GST group, which uses a tyrosine residue to activate glutathione, and the S/C-GST, which instead uses serine or cysteine residues.
The primary role of GSTs is to detoxify xenobiotics, thereby preventing their interaction with crucial cellular proteins and nucleic acids. Specifically, the function of GSTs in this role is twofold: to bind both the substrate at the enzyme's hydrophobic H-site and GSH at the adjacent, hydrophilic G-site, which together form the active site of the enzyme; and subsequently to activate the thiol group of GSH, enabling the nucleophilic attack upon the substrate. Both subunits of the GST dimer, whether hetero- or homodimeric in nature, contain a single nonsubstrate binding site, as well as a GSH-binding site. In heterodimeric GST complexes such as those formed by the cytosolic mu and alpha classes, however, the cleft between the two subunits provides an additional high-affinity nonsubstrate xenobiotic binding site, which may account for the enzymes' ability to form heterodimers. |