|
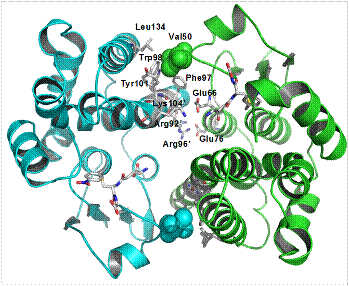
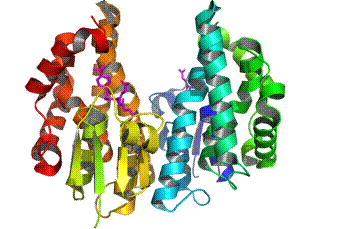
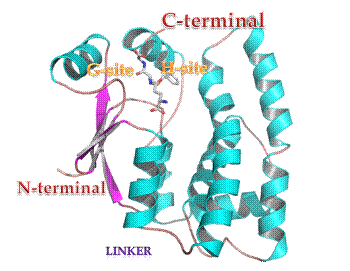
The glutathione transferases (GSTs, EC 2.5.1.18) are multifunctional enzymes that catalyse the nucleophilic attack of the thiol group of the tripeptide glutathione (γ-Glu-Cys-Gly, GSH) toward a wide range of electrophilic xenobiotic compounds. GSTs in both animals and plants are composed of two subunits, each with molecular masses in the range of 25-27 kDa and are either homodimers of a single gene product or heterodimers of subunits encoded by different but related genes.
Since many of GST derived substrates are potential cytotoxins, mutagens and carcinogens, this metabolic pathway constitutes an important cellular defense mechanism. In plants, molecules that have been conjugated to GSH are efficiently imported into the vacuole via ATP-binding cassette transporters (GS-X pumps), before being further metabolized into a range of known sulphur-containing metabolites. This import of GSH-conjugated compounds into the vacuole, acts to limit the effects of GST end-product inhibition and to further protect plant cells from danger by sequestration of those compounds whose conjugation with GSH does not cause detoxification.
In the GSTs, we, therefore, have a diverse superfamily of proteins which in the course of their evolution, have assumed diverse functions. In both animals and plants, these proteins have long been associated with stress tolerance. Indeed, a conserved feature of these proteins is their inducibility by diverse biotic and abiotic stress treatments, including exposure to toxic chemicals, environmental stress and disease. In most cases the protective action of GSTs can be shown to directly result from their ability to catalyse the glutathione conjugation and, hence, detoxification of foreign toxins. In addition, GSTs are also known to conjugate endogenous cytotoxins produced as a result of oxidative stress. In other cases, GSTs act as glutathione peroxidases, using glutathione as a reductant to covert organic hydroperoxides, formed during oxidative stress, to the less reactive monohydroxy alcohols. GSTs are also known to catalyze glutathione-dependent isomerisation reactions, thus altering the biological activity of both endogenous metabolites and foreign compounds. GSTs have also been shown to be important in binding cytotoxins, such as porphyrins and drugs in animals, and anthocyanins in plants. The functional diversity of these proteins continues to be discovered, with recent reports of the involvement of GSTs in stress induced signaling and preventing apoptosis.
The GST superfamily in plants has been subdivided into eight classes, seven of which (phi, tau, zeta, theta, lambda, dehydroascorbate reductase, and tetrachlorohydroquinone dehalogenase) are soluble and one is microsomal. The majority of the plant GSTs belongs to the tau (GSTU) and phi (GSTF) classes, which are plant specific. GSTs are present in all plant tissues and at all stages of plant development from early embryogenesis to senescence. An established role of plant GSTs is their involvement in herbicide detoxification. In addition, they have been implicated in defence mechanisms against other stress responses such as pathogenic attack, oxidative stress, heavy-metal toxicity and the normal metabolism and biosynthesis of secondary plant products. Plant GSTs are characterized and differentiated by broad and partial overlapping substrate specificity, heterogeneity of subunit composition and amino acid sequences, and differential regulation by stresses. |
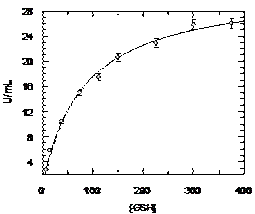
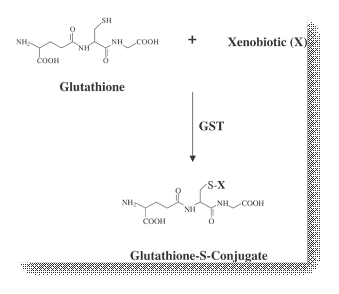
|
Glutathione transferases |